Tamoxifen 10 mg 50 Tablets Teva
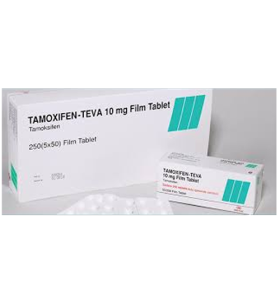
- Brand: TEVA
⚠️ WARNING: Potential Risks of Anabolic Steroids
Anabolic steroids can cause hormonal imbalances, liver strain, cardiovascular issues, and psychological effects, and should only be used under strict medical supervision. Unauthorized or improper use may lead to serious health complications.
If used, obtain these substances exclusively from licensed, verified medical providers to ensure safety and proper oversight.
What is Tamoxifen 10 mg 50 Tablets Teva?
Manufacturer: TEVA, Turkey
Substance: Tamoxifen Citrate
Pack: 1 x 50 tabs (10 mg/tab)
Pharmacological properties:
Indications:
Breast Cancer
Adjuvant therapy: Tamoxifen is indicated after total mastectomy or segmental mastectomy, axillary dissection and radiotherapy in breast cancer women without axillary lymphodenopathy. Tamoxifen is also indicated in postmenopausal women with lymphodenopathy after total mastectomy or segmental matectomy, axillary dissection and radiotherapy.
Advanced disease Treatment: Tamoxifen is effective in the treatment of advanced breast cancer in women. Tamoxifen is an alternative to ophorectomy or ovarian irradiation in women with premenopausal breast cancer.
Contraindications:
It is contraindicated in pregnant women who are hypersensitive to tamoxifen.
Warnings / Precautions:
Tamoxifen should be used under the supervision of a physician experienced in hormonal cancer chemotherapy. Premenopausal women taking tamoxifen are at risk of becoming pregnant because Tamoxifen causes ovulation. Visual disturbances such as changes in the comea, cataracts and retinopathy were observed. Hypercalcemia has been reported in breast cancer patients with bone metastases when combined with estrogens and adrogens. Appropriate precautions are taken and Tamoxifen treatment is discontinued if necessary. Once hypercalcemia is controlled, treatment may be resumed under reduced dose or low dose prednisone.
An increase in the occurrence of endometrial changes such as hyperolasis, polyp and endometrial cancer has been observed in tamoxifen treatment. Previously used or currently using Tamoxifen; Patients with abnormal vaginal bleeding, menstrual irregularity, vaginal discharge and pain and pressure on the pelvis should be examined immediately. Tamoxifen should be used with caution in harvesting with leukopenia and thrombocytopenia. Periodic whole blood counts including erythrocyte are recommended in patients using the drug. Periodic liver function tests should be performed. Periodic serum triglyceride and cholesterol measurements are recommended in patients with hyperlipoproteinemia. Periodic liver function tests should be performed. Periodic serum trglyceride and cholesterol measurements are recommended in patients with hyperlipoproteinemia. If abnormal vaginal bleeding occurs, this should be reported to the physician and should be investigated. Tamoxifen has been reported to be carcinogenic in animals, although the findings in humans are not sufficient. The potential carcinogenic potential for humans should be considered when taking tamoxifen.
Use in Pregnancy and Lactation: tamoxifen shouldn’t be used while pregnancy. If the drug is used during pregnancy or becomes pregnant while using the drug, the patient should be informed of potential risks, including spontaneous abortion, birth defects, fetal deaths and vaginal bleeding. It is recommended not to use tamoxifen during lactation.
Side effects / Adverse effects:
Adverse reactions due to tamoxifen are mild or infrequent and do not require discontinuation of treatment. If adverse reactions are severe, they can be controlled with a simple dose reduction without losing control of the disease. If side effects do not respond, discontinue of the treatment may be necessary. Side effects during long-term treatment with tamoxifen are not as severe as the side effects of androgens and estrogens used in the treatment of breast cancer. The drug is classified as antiestrogenic effect (redness, vaginal, bleeding, pruritus vulva) or general effects (gastrointestinal intolerance, tumor pain, dizziness, skin rash) and rare effects (fluid reaturation and partial hair loss). Tamoxifen causes changes in liver enzyme levels and causes liver disorders such as liver fat, cholestasis, hepatitis and hepatic necrosis. Hot flashes are nausea and / or vomiting. These reactions may occur in 25% of patients. Less frequently reported adverse reactions are: Grenitourinary system; vaginal bleeding and discharge, menstrual irregularities Bone and soft tissue; Severe bone and tumor pain may occur during tamoxifen treatment and sometimes these effects are associated with a good tumor response. Analgesics may be needed for pain. Patients with soft tissue disease may have an increase in the size of pre-existing lesions. This increase is sometimes accompanied by significant erythema in and around the lesion, and may also occur new lesions. Bone pain burns appear and disappear immediately after the start of treatment. Adverse reactions reported quarterly; Thromboembolic findings (deep vein thrombosis, pulmonary embolism, superficial phlebitis) have been reported rarely during tamoxifen treatment. It is known that these findings occur in patients with increased tumor formation. The causative relationship of tamoxifen has not been established. Menstruation of premono-posal women using tamoxifen for breast cancer treatment is prevented. Reversible ovarian cysts were observed in women treated with 40 mg Tamoxifen twice daily for short term.
Other infrequent adverse reactions; Hypercalcemia, peripheral edema, non-taste of food, pruritus vulva, depression, dizziness, and headache.
Drug interactions:
Tamoxifen / Anticoagulants; When tamoxifen is used in combination with coumadine type coagulants (eg Warfarin), there may be a significant increase in anticoagulant effect. To compensate for safe anticoagulation, a significant reduction in the daily dose of anticoagulant may be necessary. Life-threatening interactions have been reported. Tamoxifen / Antineoplastics, Tamoxifen, N-desmethyl tamoxifen c and 4-hydroxytamoxifene were found to be potent inhibitors of liver cytochrome p 450enzyme system. The effect of Tamoxifen on the metabolism and excretion of cyclophodfamide and other antineoplastic drugs that require oxidase enzyme system for effect is not known.
Dosage and administration:
Twice a day (morning and evening) 10-20 mg. The first response to treatment is usually achieved 1-3 months after onset. A daily dose of more than 20 mg is beneficial in cases where the disease progresses and treatment is passed.
Warning!
Using anabolic steroids with or without any knowledge about the subject can do harm to your body. They even can cause death. You have to be careful about getting checked before, during and after using anabolic steroids. Our firm warns you about the all of the risks of using steroids and we do not accept any responsibility about health issues and consequences. Therefore we do not sell who are not over 20 years old.